Research Overview
Research in the Best group entails bio-organic, synthetic organic, medicinal and supramolecular chemistry. This generally involves the design, synthesis and study of organic molecules for applications pertaining to biological systems. Our lab has a particular emphasis on lipid membranes, which are at the forefront of biomedical research since lipids control critical biological pathways relevant to disease, and are beneficial for applications such as drug delivery. One area of research in the group involves the development of smart liposomes for drug delivery through the design of responsive lipid structures. Additionally, we develop functionalized lipid probes that can be applied to elucidate the roles of lipids in biological processes. These projects utilize synthetic organic chemistry to access designer lipid targets, followed by biological and analytical chemistry studies to study and apply these compounds.
Smart Liposomes for Drug Delivery
Liposomes are promising drug carriers that can be used to encapsulate and enhance the delivery of a range of medicinal agents with different properties. Current goals for the enhancement of liposome delivery include expanding the ability to selectively target liposomes to diseased cells, and controlling release of encapsulated drugs at the desired time and location. We have worked to address these issues by developing customized lipids by which liposome properties can be manipulated. In particular, we have developed synthetic switchable lipid analogs that undergo conformational changes in the presence of external stimuli that alter membrane self-assembly properties and trigger the release of contents (Figure 1). Toward this end, we have developed liposomes that undergo cargo release when subjected to light, calcium and carbohydrates. Each of these targets is applicable for the development of advanced liposomal drug delivery platforms with control over the site of drug delivery.
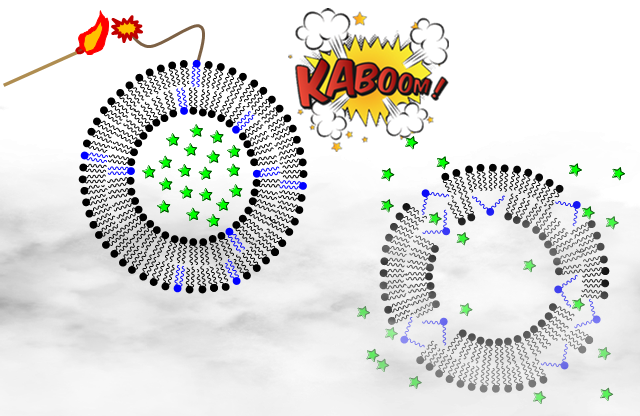
Figure 1. Cartoon depicting triggered release from liposomes
For a representative example, we have developed liposomes that release contents in the presence of calcium (Figure 2A). This is an important target for medicinal applications since calcium concentrations are significantly elevated in cells infected with malaria plasmodia. This is also an example of a new paradigm for controlled release we have pioneered in which cargo release is triggered by the recognition of specific chemical metabolites or ions, for which concentrations typically fluctuate in diseased cells. To induce calcium-responsive properties, we designed and synthesized lipid 1, which contains a polar calcium-binding domain as well as hydrophobic lipid chains to produce an amphiphilic structure (Figure 2B). This compound was designed such that calcium binding causes constriction of the sensing headgroup, which disturbs the packing of this lipid in the membrane, stimulating content release. We employed fluorescence-based release studies, dynamic light scattering and scanning transmission electron microscopy to characterize content release and membrane reorganization upon titration with calcium.
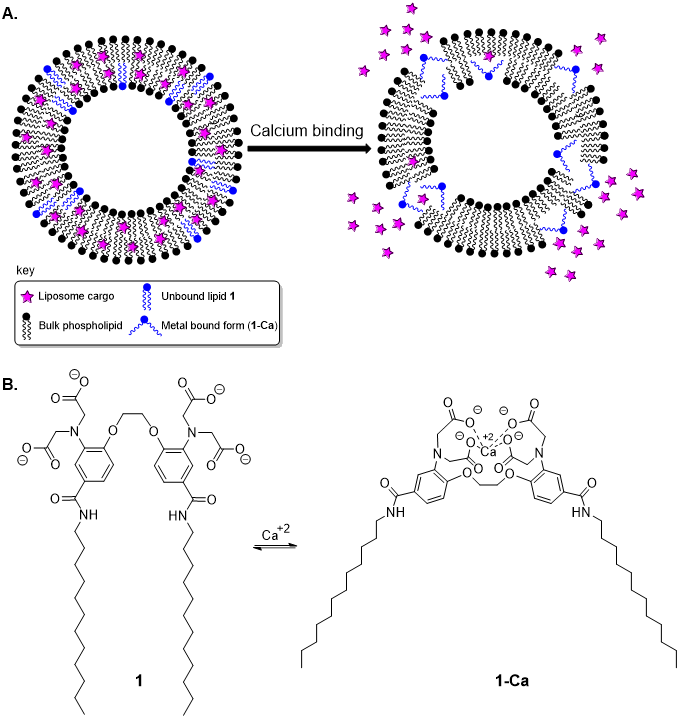
Figure 2. Calcium-responsive liposomes driven by lipid conformational changes upon calcium binding.
Lipid Probes for Investigating Lipid Biological Activities
The determination that lipids control many critical biological processes and pathways is a relatively recent advancement. As a result, many diseases are caused by dysregulation of the abundance or activity of key lipid molecules. Therefore, synthetic lipid probe molecules are invaluable for interrogating the biological roles of lipids and analyzing variations in their abundance between healthy and diseased cells. Our group has developed and applied lipid probes for a range of biological studies including the metabolic labeling of lipids to visualize lipid biosynthesis in cells, bifunctional lipid activity probes for the discovery of proteins that bind to and are regulated by lipids, and high-throughput microarray assays to analyze lipid and membrane binding properties.
For a representative example, we have developed analogs of lipid-producing substrates containing “clickable” tags for the metabolic labeling of lipid products (Figure 3). The diminutive tag on these substrate analogs allows them to infiltrate biosynthetic pathways, thereby producing tagged versions of their native lipid products, which can be modified after the fact through click chemistry. Following design and synthesis, we study the ability of these tagged substrate analogs to hijack lipid biosynthesis and produce labeled products through studies including cellular fluorescence microscopy, competition against native enzyme substrates, mass spectrometry- and thin layer chromatography-based detection of labeled products, and cell growth assays. These techniques allow for the direct visualization of lipid products in cells, and are being applied to study how lipid biosynthesis is altered in diseased cells.

Figure 3. metabolic labeling of lipid products in cells and detection of product incorporation